46
@
U S T . H K
The causes of Alzheimer’s disease can be roughly grouped into three
categories (shaded ovals): cellular events (light green), genetic events
(blue) and molecular events (dark green), and the various elements
interact with each other. Thus, for example, inflammation can enhance
the deposition of beta-amyloid peptides, which in turn can influence the
deposition of tau and impair synaptic function.
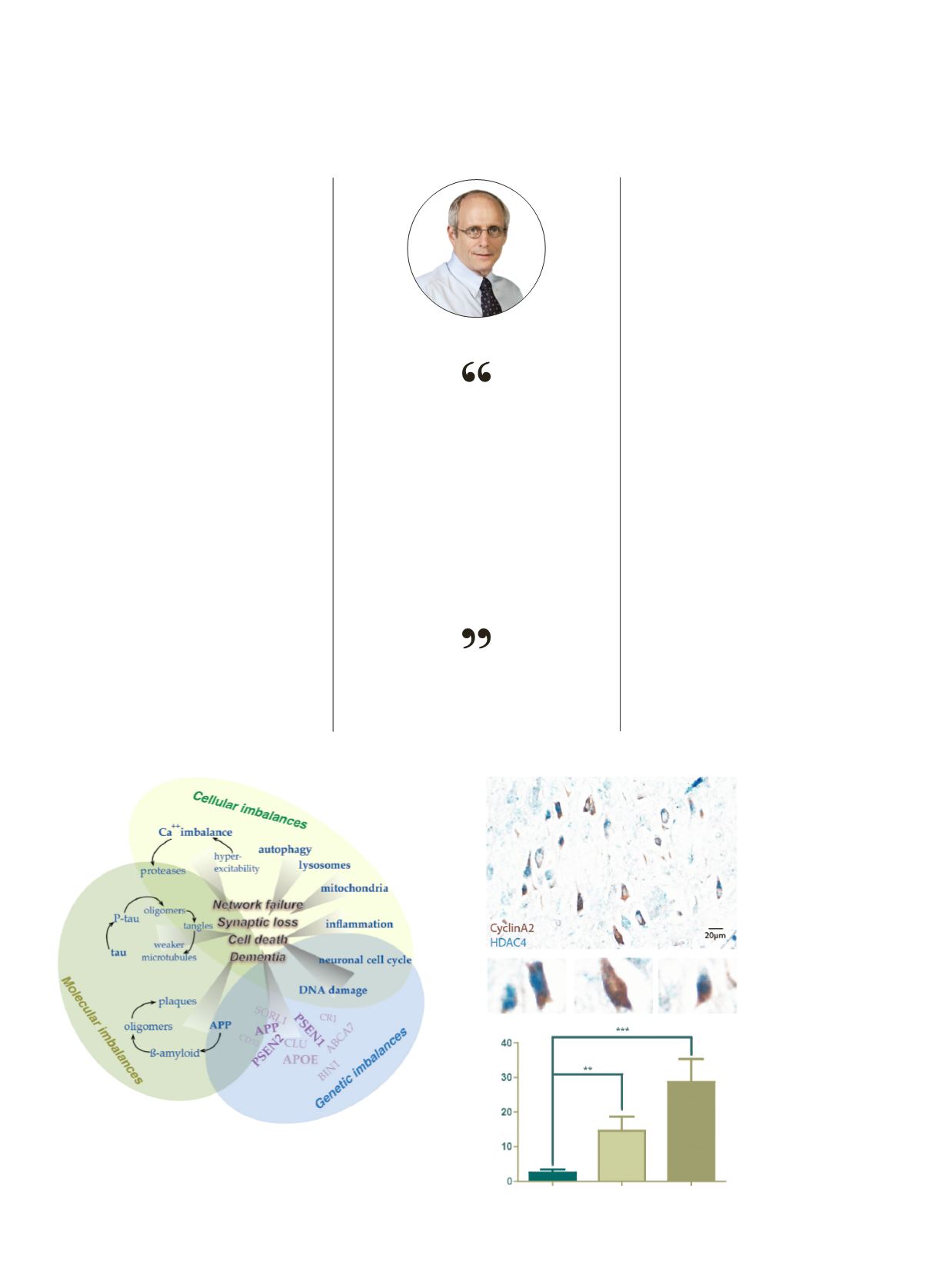
The brown stains show
proteins that are normally
only found in dividing cells.
Their presence in these
neurons is a sure sign that
something is not right.
The blue stain, a separate
protein HDAC4, moves to
the nucleus when a cell
tries to divide. Normal
neurons cannot divide, but
in Alzheimer’s diseased
patient the neurons try.
This attempt will probably
kill them. The histogram
shows that as Alzheimer’s
progresses, the fraction of
cells that are trying to divide
increases.
Mild cognitive
impairment
CyclinA2 neurons (%)
Normal
Alzheimer’s
disease
Aging vs Amyloid
Prof Karl Herrup has championed an
alternative hypothesis that emphasizes
aging rather than amyloid as the key con-
tributor to the disease. His work focuses
on the biology underlying the process of
cell death that occurs during the course
of Alzheimer’s disease, searching for
the molecular triggers that start the cell
death, and the strategies we can use to try
to prevent it.
The genesis of human neurons stops
almost completely by one year of age;
after that, mature adult neurons are in-
capable of cell division. Yet in the regions
of the Alzheimer’s disease brain where
cell death occurs, Prof Herrup and his
team have shown that neurons are trying
to do the impossible. They are trying to
divide. Prof Herrup has been responsi-
ble for identifying the molecular details
of why this happens. His search for the
signals that fool the cells into making
what is essentially a lethal move has led
to some remarkable findings. He sees
the initiation of cell division as a good,
but ultimately fatal instinct. “Neurons
sense damage and like skin cells trying
to heal a cut, their instinct is to increase
PROF KARL HERRUP
Chair Professor and Head, Division of Life Science,
Co-director, HKUST Super-Resolution Imaging Center
We can conceive of
Alzheimer’s disease as aging
plus an injury that triggers
a decline and a cascade of
events. It is not normal aging,
but you don’t need the amyloid
peptide to create it
their numbers by trying to divide,” he
said. “But this is not possible in an adult
neuronand the instinct goes sour on them.
I call it divide and die.”
What forces an adult neuron into
this situation? Prof Herrup and his team
have pioneered work implicating an
abnormal immune response. The brain
of the person with Alzheimer’s has long
been recognized as being in a state of
chronic inflammation. Both genetic and
epidemiological studies point to the
importance of this process and the roles
that the immune system and neural
inflammation can play in modulating
neurodegenerative disease.
Over the years, this broad view of
the origins of the cells death has led Prof
Herrup to raise questions about and
ultimately challenge the most prevalent
disease model of Alzheimer’s – the so-
called amyloid cascade hypothesis, which
regards the accumulation of beta-amyloid
peptides as the root cause of Alzheimer’s
disease. His views on the topic were re-
cently published in
Nature Neuroscience
.
This distinction has important implica-
tions for future research – both basic bi-
ological science as well as clinical trials.


